Description
** All Rapid Tests are FINAL SALE – we cannot offer cancellations or refunds after your order has shipped. **
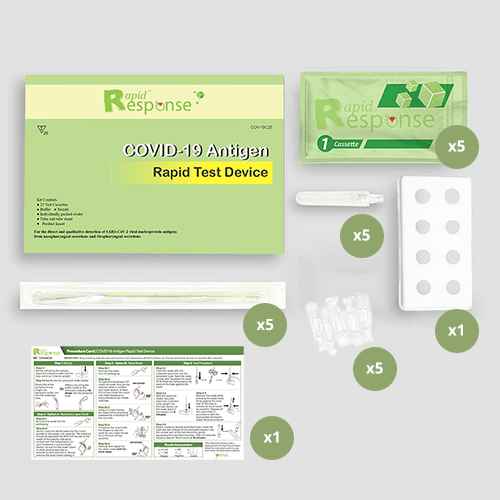
Each Box Contains:
| 5-pack | 25-pack |
| 5 Test cassettes | 25 Test cassettes |
| 5 Individually packed swabs | 25 Individually packed swabs |
| 5 Individually packed buffers | 2 Bottles of extra buffer liquid |
| 5 Tubes and nozzles | 25 Tube and Nozzles |
| 1 Tube stand | 1 Tube stand |
| 1 Product insert | 1 Product insert |
Specification:
- Sample Type: Nasal and Nasopharyngeal swab
- Detection Method: Colloidal Gold
- Detection Time: 15 minutes
- Relative Sensitivity: 95.60 % for NP swab; 90.20% for nasal swab
- Relative Specificity: 100 % for NP swab; 100% for nasal swab
- Interim Order (IO#321669)
- All necessary reagents provided & no equipment needed
DISCLAIMER – READ BEFORE CHECKOUT
The BTNX Rapid Response COVID-19 Antigen Test is authorized for healthcare professional use by Health Canada (Interim Authorization Number: 321669). Self-swabbing by trained individuals is currently permitted in certain provinces (Ontario, Alberta, British Columbia, and Saskatchewan). Before Rapid Testing kits can be purchased, the following training materials and documents must be reviewed:
- BTNX COVID-19 Antigen Rapid Testing Device Instructional Video
- BTNX Rapid Response Product Manual
- BTNX Rapid Response Procedure Card
Rapid antigen testing devices should only be used for screening purposes. Nucleic acid-based testing, also called molecular testing or PCR, is the gold standard to diagnose active COVID-19 infection in patients with symptoms.