Description
** CLIA number Required. All Rapid Tests are FINAL SALE – we cannot offer cancellations or refunds after your order has shipped. **
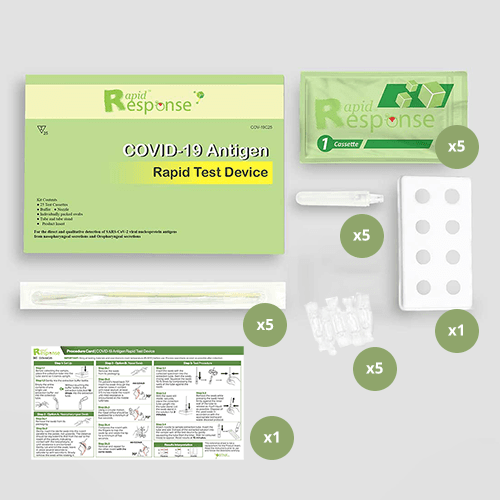
Each Box Contains:
- Collection Swabs: 25 sterile nasal swabs
- SARS-CoV-2 Buffer: 25 single-use vials of solution containing 5 mL of buffer
- Transfer Pipette: 25 single-use, fixed volume pipettes
- Accula SARS-CoV-2 Test Cassette: 25 Single-use, foil-pouched with desiccant and Test Cassette
- SARS-CoV-2 High Positive Control Swab: DNA Based Synthetic Oligo dried onto a swab well-above the limit of detection
- SARS-CoV-2 Low Positive Control Swab: DNA Based Synthetic Oligo dried onto a swab near the limit of detection
- SARS-CoV-2 Negative Control Swab: Buffer solution dried onto a swab
- Documentation: 1 Self-collection Quick Reference and 1 Electronic Instructions for Use (EIFU) card
Specification:
- Test type: Molecular in vitro diagnostic test utilizing polymerase chain reaction (PCR) and lateral flow technologies for the qualitative, visual detection of the coronavirus SARS-CoV-2 viral RNA.
- Specimen type: Nasal
- Turnaround time: Approximately 30 minutes