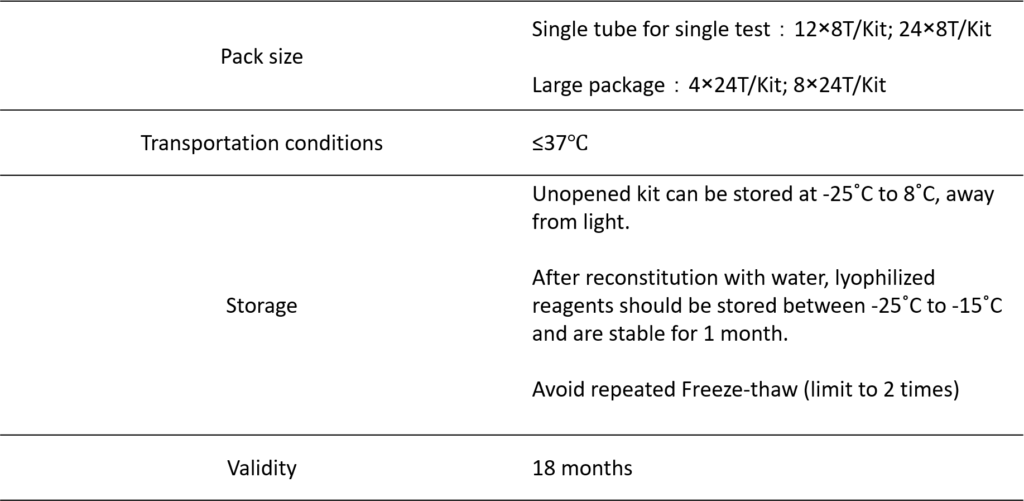
PRODUCT INFORMATION
The 2019-nCoV/IAV/IBV Nucleic Acid Test Kit has been developed for the detection of SARS-CoV-2, Influenza A and Influenza B RNA from human nasopharyngeal swabs, oropharyngeal swabs, sputum, alveolar lavage fluid specimens.
The kit is CE-certified and intended for in vitro diagnostic use only.
The SARS CoV2/IAV/IBV PCR kit is a one-step real-time reverse transcription polymerase chain reaction (RT-PCR) diagnostic kit. Used for the detection of SARS-CoV-2 as well as Influenza A and B.
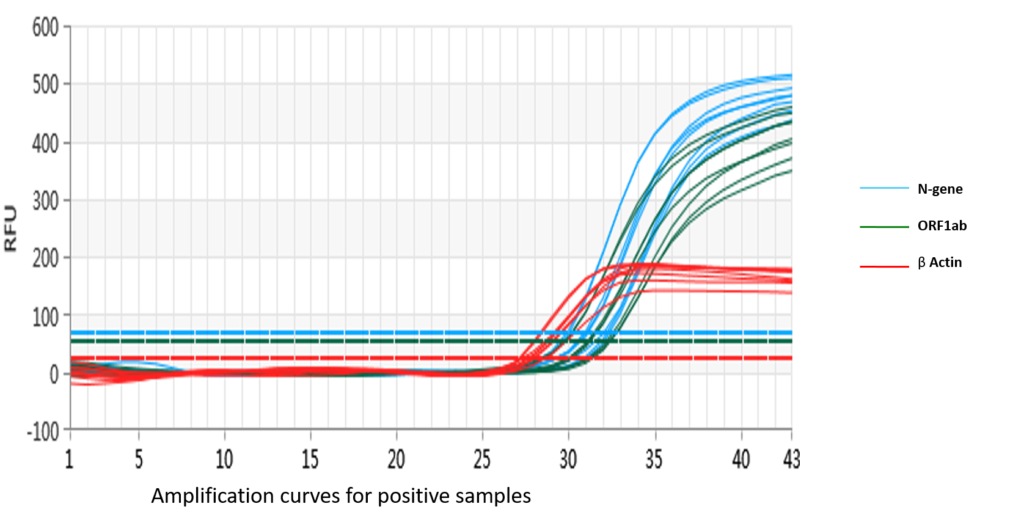
The multiplex assay uses fluorescent probes and primers to detect three specific regions within the SARS-CoV-2 nucleocapsid (N) gene and the ORF1ab gene. β-globulin is used as an internal control to monitor the quality of the specimen and extraction process. Positive and negative controls are included. RNA isolated from patient samples can be directly used in the assay to be reverse transcribed and amplified quantitatively. The pseudoviral particles used as positive control act as a control for the extraction process as well as the RT-PCR.
| Components | Main Ingredients | |
| 1 | SARS-CoV-2 reaction mixture, lyophilized | Primers, probes, PCR reaction buffer, dNTPs, Enzyme, etc. |
| 2 | SARS-CoV-2 positive control, lyophilized | Lyophilized pseudoviral particles including ORF1ab and N genes detecting target sequence |
| 3 | Negative control | Ultrapure water |
This test kit can be used to detect SARS-CoV2
Influenza A virus (2009 H1N1, H1N1, H3N2, H5N1, H7N9) and Influenza B virus (Yamagata, Victoria) specifically.
FAM channel detects SARS-CoV-2, HEX channel detects Influenza A, Cy5 channel detects Influenza B, ROX channel detects the human internal reference gene as quality control.
- SARS-CoV2 (2019-nCoV ): 400 copies/mL.
- Influenza A virus: 1.5 TCID50/mL.
- Influenza B virus: 2.0 TCID50/mL.
SARS-CoV2/IAV/IBV One-Step PCR amplification plot