PRODUCT INFORMATION
The HYRIS bKITTM Direct COVID-19 is a real-time RT-PCR assay intended to be used as near-patient testing on the HYRIS bCUBETM instrument and HYRIS bAPPTM for the in-vitro diagnostic qualitative detection of SARS-Cov-2 nucleic acid in human upper respiratory tract specimens such as, nasopharyngeal swabs (NPS), nasal swabs (NS), and a combination of oropharyngeal swabs (OPS) and NPS, and oropharyngeal and NS specimens collected by a healthcare professional from individuals suspected of COVID-19 disease.
Hyris System (including bKIT, bCUBE and corresponding software) is a smart, efficient and effective solution for COVID-19 testing which has both CE IVD and Health Canada Approval.
Current detection coverage of the following new variants is 100% by the Hyris system:
- Alfa variant known as B.1.1.7/501Y.V1
- Beta variant known as B.1.351/501Y.V2
- Gamma variant known as P.1/501Y.V3
- Delta variant known as B.1.617
- Omicron variants known as B.1.1.529 and BA.5
- Californian variants known as B.1.429 and B.1.427
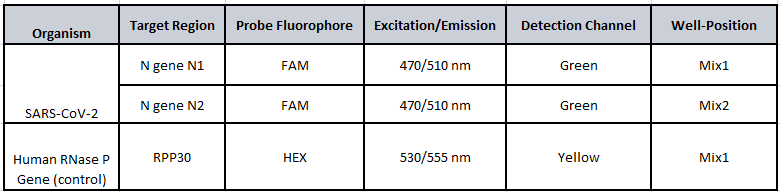
Workflow Diagram:
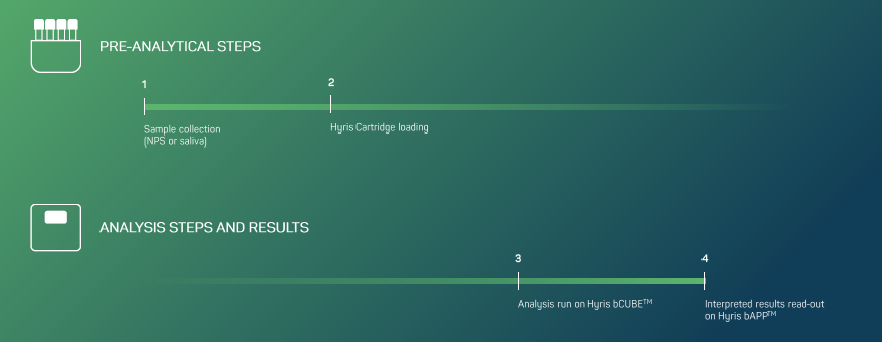
Reported Genes and Corresponding Channel: