PRODUCT INFORMATION
The COVID-19 Antibody Rapid Test is a lateral flow in-vitro immunoassay intended to detect the presence of SARS-CoV-2 IgM and IgG antibodies instantly from a whole blood sample collected from a minimally invasive fingerstick blood collection process.
Fastep COVID-19 IgM/IgG Rapid
Antibody Test
Each Box Contains
- 20 Test Devices
- 20 Disposable Pipettes
- 20 Alcohol Prep Pads
- 20 Sterile Safety Lancets
- 1 Sample diluent
- 1 Instructions for use (IFU)
Specification:
- Sample Type: Fingertip blood, whole blood sample, serum and plasma
- Detection Method: Colloidal Gold
- Detection Time: 15 minutes
- IgM sensitivity: 100.0%, specificity: 98.8%
- IgG sensitivity: 90.0%, specificity: 100.0%.
- US FDA-EUA approved and CE certified ; Interim order: IO#312782
INSTI
COVID-19 Antibody Test

Each Box Contains
- 1 Membrane Unit
- 1 Sample Diluent
- 1 Colour Developer
- 1 Clarifying Solution
- 1 Pipette
- 1 Lancet
- 1 Alcohol Swab
- 1 Package Insert
Specification:
- Test Technology: IgG/IgM (qualitative)
- Diagnostic Sensitivity: 98.2%
- Diagnostic Specificity: 99.6%
- Storage Temperature: 2-30°C/36-86°F
- Time to Result: 1 minute
- Sample Collection:Blood
- US FDA-EUA approved and CE certified ; Interim order: IO#326459
RESULTS AND INTERPRETATION
Want more detail on how to interpret the COVID-19 IgM/IgG Antibody Rapid Test? See our article here!
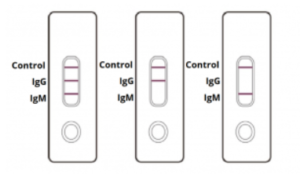
IgG and IgM POSITIVE (left): Both the test lines and the quality control line are colored in the COVID-19 IgM/IgG Antibody Test Cassette.
IgG POSITIVE (middle): Two lines appear on the COVID-19 IgM/IgG Antibody Test Cassette. One coloured line appears in the control line region, and another coloured line appears in the IgG test line region. The result is positive for SARS-CoV-2 specific-IgG antibodies.
IgM POSITIVE (right): Two lines appear on the COVID-19 IgM/IgG Antibody Test Cassette. One coloured line appears in the control line region, and another coloured line appears in the IgM test line region. The result is positive for SARS-CoV-2 specific-IgM antibodies.
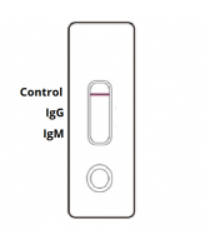
INVALID: Control line fails to appear. Insufficient sample volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette.
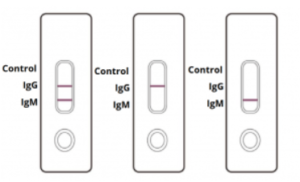
NEGATIVE: One coloured line appears in the control region. No apparent coloured line appears in the IgG or IgM test region.
LIMITATIONS
- The authorized use of this test in Canada is limited to trained healthcare professionals. This test shall not be used for self-testing or distributed or sold for home use.
- This test has been authorized by FDA under an EUA for use by authorized laboratories.
- This test has not been FDA cleared or approved.
- This test has been authorized only for the presence of IgM and IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens.
- This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
- This product is intended for professional use and not for home use.
- Not for the screening of donated blood.
To find more information about serological testing from Health Canada, click here.
Frequently Asked Questions
Pursuant to Section 5 of the Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19, made by the Minister of Health on February 18, 2020, The FaStep Rapid COVID-19 IgG/IgM Rapid Test Device and Rapid COVID-19 Antigen Test (both distributed by Aurora) are now authorized for sale or importation in Canada.
No, these rapid tests do not need any additional instruments to read the results. These test kits are self-contained, containing all the tools you need from sample collection to result reading.
The test is for use by trained laboratory or healthcare professionals. This assay is not intended for at-home testing or self-testing. Laboratories are required to report all results to the appropriate public health authorities.
This test is not for the screening of donated blood.
Yes! Aurora Biomed Inc. holds a Medical Device Establishment License from Health Canada.
Please interpret the result of rapid antigen and antibody tests within 15-30 minutes. The result is not valid if you check again in a few hours.