PRODUCT INFORMATION
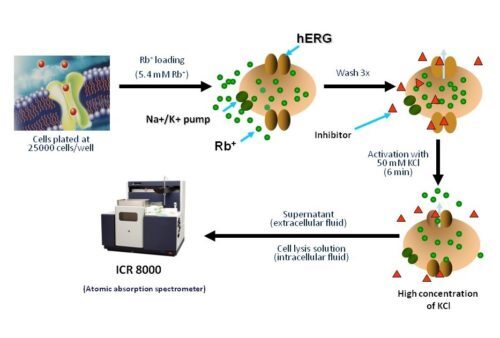
The ICR series detects ion movements across membrane proteins by measuring the concentration of intracellular and cytosolic ions of interest using Atomic Absorption Spectroscopy (AAS). This technique is independent of and complementary to methods that rely on voltage manipulation and is used to predict drug potency (IC50).
Principle
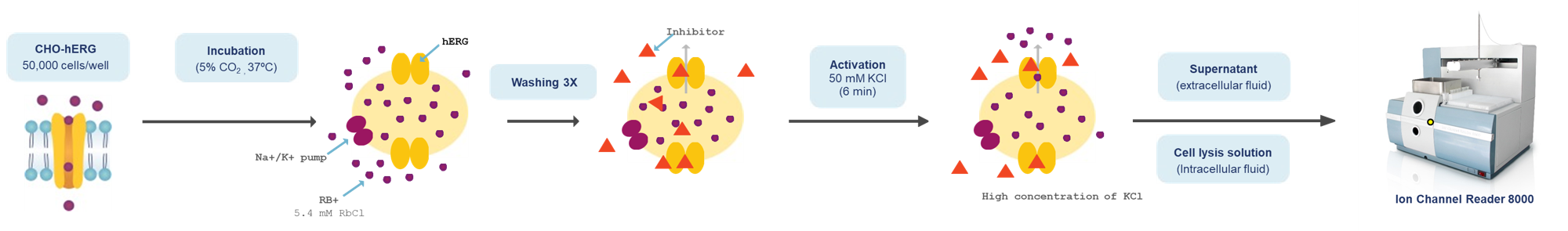
Non-radioactive Rubidium Efflux Assay
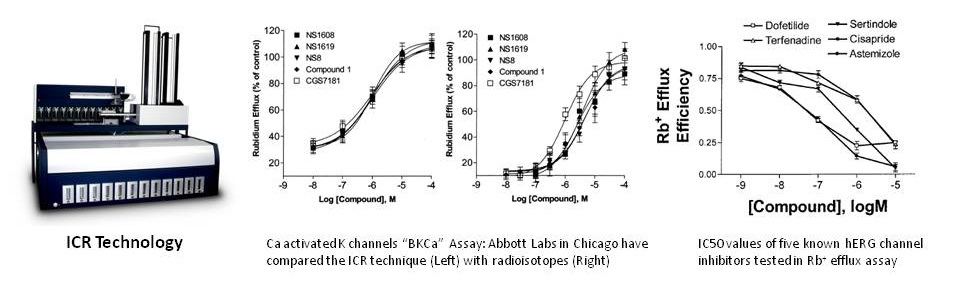
“ICR to IC50” Results from ICR 8000/12000
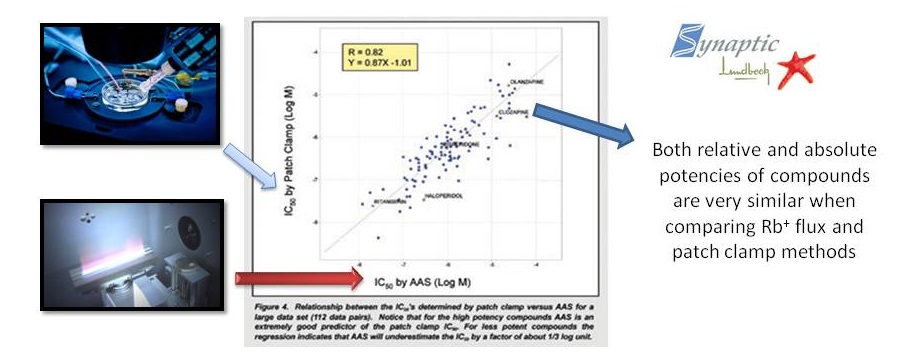
Validation: Comparision between patch-clamp and ICR on obtaining the IC50
A comparison of Efflux Assay and Manual Patch Clamp Data on hERG channels clearly shows that measurement of Rb+ efflux is a reliable and accurate method for predicting the potency of compounds (IC50 values) blocking the hERG channels.
Aurora’s Ion Channel HTS Screening Assay Uses
Stable cell lines expressing various ion channels are used to give you the best quality data to decide on advancing molecules to the next phase of drug development.
Aurora employs flux assay technology (non-radioactive ions) as tracers for specific ion channels and transporters. High-throughput screening is offered on Aurora’s ICR 8000 and ICR 12000 systems.
Drug-induced proarrhythmic risk and cardiac dysfunction assessment is critical to modern cardiac safety screening. Aurora offers an integrative approach to various functional assays for compound screening.
Frequently Asked Questions
Cells lines with stable or transient expression of the ion channels of interest are available for screening. In addition, Aurora offers cardiac functional assays that include:
• Action potential recordings from isolated tissues (rabbit Purkinje fibers, atria, guinea pig papillary muscle, hiPSC-derived cardiomyocytes, isolated primary cardiomyocytes)
• Contractility assay (sarcomere shortening of isolated cardiomyocytes using the IonOptix system)
• Rabbit Torsades de Pointe model
• Left ventricular pressure-volume analysis on anesthetized animals
We recommend testing a minimum of 4 concentrations in triplicates for generation of a dose response curve and calculation of an IC50 value.
The hERG channel is an important contributor to cardiac repolarization. But, although Inhibition of hERG channels can lead to delayed repolarization, it does not necessarily lead to arrhythmia. The cardiac action potential results from the balance of activity of multiple ionic currents. Inhibition of hERG channels can have a limited impact when other ion channels are inhibited as well. Thus, it is important to assay the effects of test compounds on hERG (IKr), but also on Nav1.5 (peak and late sodium current), Cav1.2 (calcium current, ICa,L), KCNQ1/E1 (slow delayed rectifier, IKs), Kir2.1 (inward rectifier, IK1).
The ion channel of interest is usually expressed in mammalian cells such as HEK cells and its activity is recorded using the patch clamp method or high throughput systems (e.g. Aurora’s Ion Channel Reader). New cell systems (human iPSC-derived cardiomyocytes) are now available to assess effects of compounds on ion channels in a more physiologically relevant model. Action potential recordings from hiPSC-CM and rabbit Purkinje fibers are also valuable models to predict proarrhythmic liability. They complement the ion channel data.


