PRODUCT INFORMATION
COVID-19 IgM/IgG Antibody Rapid Test Kit – FDA EUA Authorized
In response to the Coronavirus (COVID-19) pandemic, Aurora is now offering the IgM/IgG antibody rapid test kit to equip healthcare workers for rapid COVID-19 antibody detection. This COVID-19 Rapid Test Kit is suitable for the qualitative detection of SARS-CoV-2 IgM/IgG antibodies in human serum, plasma, or whole blood. Common signs of infection with SARS-CoV-2 include respiratory symptoms, fever, cough, shortness of breath, and dyspnea. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and even death. SARS-CoV-2 can be excreted through respiratory secretions or transmitted through oral fluids, sneezing, physical contact, and through air droplets.
Healgen COVID-19 Antibody Rapid Test

Each Box Contains:
25x Individual sealed pouches (1x Test cassette, 1x Desiccant pouch), 25x disposable pipettes, sample diluent and instructions for use (IFU).
Specification:
- IgG sensitivity: 96.7%, specificity: 98.0%. IgM sensitivity: 86.7%, specificity: 99.0%.
- Overall positive agreement rate of 96.7%, negative agreement rate of 97.0%.
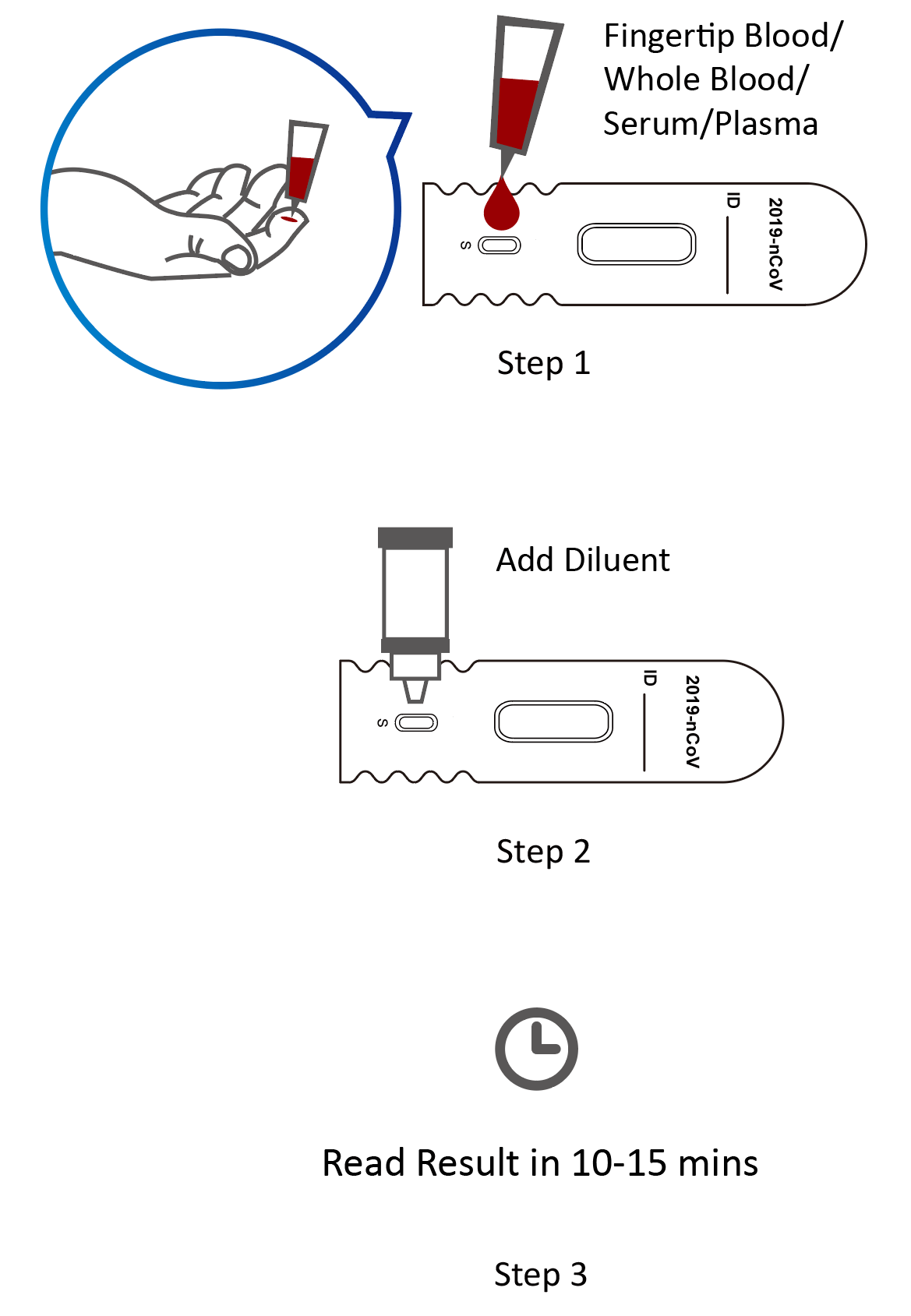
- Sample Type: whole blood sample, serum and plasma
- Detection Method: Colloidal Gold
- Detection Time: 10 – 15 minutes
- Not suitable for Point of Care Testing
- FDA EUA approved; CE Certified
FaStep COVID-19 Antibody Rapid Test

Each Box Contains:
20x Test Devices, 20x Disposable Pipettes, 20x Alcohol Prep Pads, 20x Sterile Safety Lancets, sample diluent and instructions for use (IFU).
Specification:
- IgG sensitivity: 90.0%, specificity: 100.0%. IgM sensitivity: 100.0%, specificity: 98.8%.
- Overall positive agreement rate of 80.8%, negative agreement rate of 100.0%.
- Sample Type: Fingertip blood, whole blood sample, serum and plasma
- Detection Method: Colloidal Gold
- Detection Time: 10 – 15 minutes
- Suitable for Point of Care Testing, CLIA waived
- FDA EUA approved; CE Certified
Packaging Configuration:
- 20 tests per kit, 640 tests per case, 12,800 tests per pallet
EcoTest COVID-19 Antibody Rapid Test

Each Box Contains:
20x Covid-19 Antibody Tests Cassette, 20x Sterile Fingerstick Lancets, 20x Sterile Alcohol Pads, 20x Droppers for Blood Collection, 20x Vials with Buffer
Specification:
- IgG sensitivity: 98.8%, specificity: 98.7%. IgM sensitivity: 93.7%, specificity: 99.1%.
- Overall positive agreement rate of 80.8%, negative agreement rate of 100.0%.
- Sample Type: Fingertip blood, whole blood sample, serum and plasma
- Detection Method: Colloidal Gold
- Detection Time: 15 minutes
- Suitable for Point of Care Testing, CLIA waived
- FDA EUA approved; CE Certified
Packaging Configuration:
- 20 tests per kit
How Does COVID-19 IgM IgG Rapid Test Kit Work?
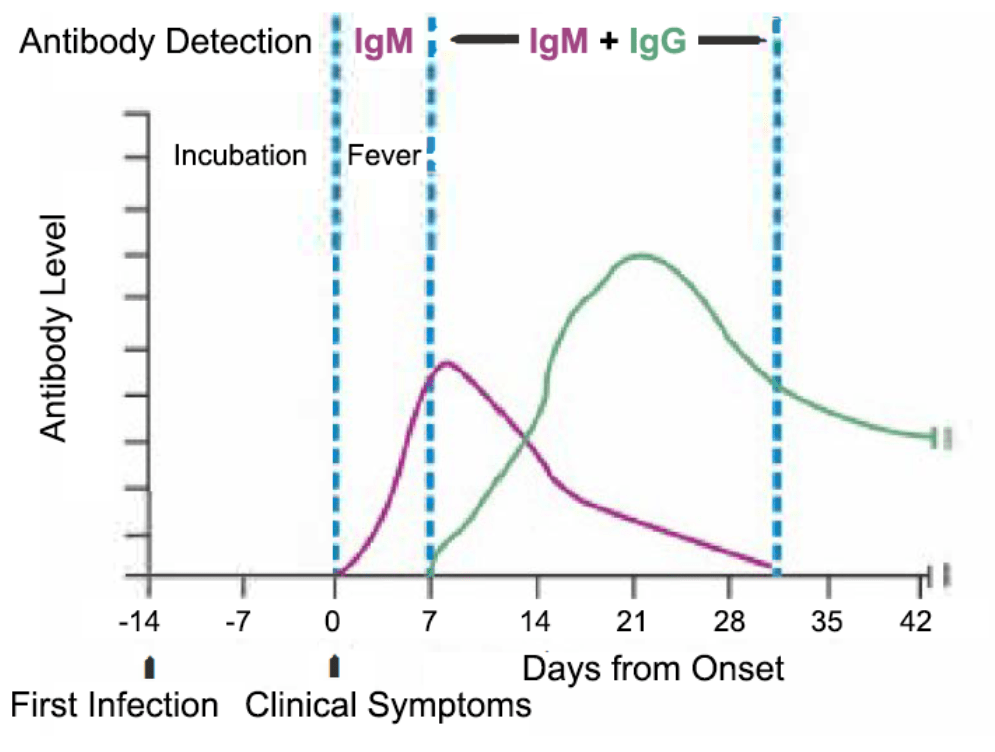
The COVID-19 IgM/IgG antibody rapid test is a fast and effective method for screening IgM and IgG antibodies against SARS-CoV-2. This test can also suggest information on the stage of infection.
Both Immunoglobulin M (IgM) and Immunoglobulin G (IgG) antibodies are produced during the primary immune response. As the body’s largest antibody, IgM is the first antibody to appear in response to an initial exposure to antigens. IgM provides the first line of defence during viral infections, followed by the generation of adaptive, high affinity Immunoglobulin G (IgG) responses for long-term immunity and immunological memory. IgG is usually detectable about 7 days after first sign of clinical symptoms.
Instructions for Use
Interpretation of Test Results
For more information on interpreting test results, see our article here.
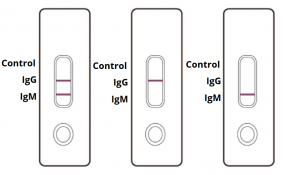
IgG and IgM POSITIVE (left): Both the test lines and the quality control line are colored in the COVID-19 IgM/IgG Antibody Test Cassette.
IgG POSITIVE (middle): Two lines appear on the COVID-19 IgM/IgG Antibody Test Cassette. One coloured line appears in the control line region, and another coloured line appears in the IgG test line region. The result is positive for SARS-CoV-2 specific-IgG antibodies.
IgM POSITIVE (right): Two lines appear on the COVID-19 IgM/IgG Antibody Test Cassette. One coloured line appears in the control line region, and another coloured line appears in the IgM test line region. The result is positive for SARS-CoV-2 specific-IgM antibodies.
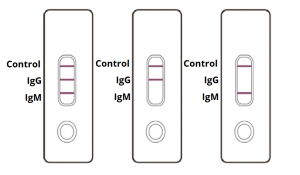
NEGATIVE: One coloured line appears in the control region. No apparent coloured line appears in the IgG or IgM test region.
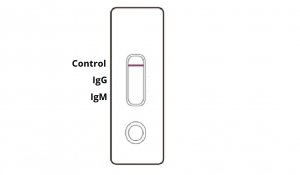
INVALID: Control line fails to appear. Insufficient sample volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the COVID-19 rapid test kit immediately and contact your local distributor.
Disclaimer: (The picture is for representation only, markings on test cassette may vary.)
Limitation of COVID-19 IgM/IgG Antibody Rapid Test
- The test is designed only for use with human serum, plasma, or whole blood samples for the qualitative detection of SARS-CoV-2 IgM and IgG antibody.
- As in case of all diagnostic tests, a definitive clinical diagnosis should not be based on the result of a single test but should rather be made after all the clinical findings have been evaluated and should be confirmed by other conventional detection methods, such as molecular diagnostics and CT.
- A false negative may occur if the amount of SARS-CoV-2 IgM or IgG antibody is below the detection level of the kit.
- If the test gets wet prior to use, or is stored improperly, it may cause incorrect results.
- The test is for qualitative detection of SARS-CoV-2 IgM or IgG antibody in human serum, plasma, or blood samples, it does not indicate the quantity of the antibodies present.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
- This test is intended for healthcare professional use and not for home use.
- This test is not for the screening of donated blood.
Warnings
- This test has been authorized by FDA under an EUA for use by authorized laboratories.
- This test has not been FDA cleared or approved.
- This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Frequently Asked Questions
The Real-Time RT-PCR (nucleic acid test) for SARS-CoV-2 is based on the detection of viral RNA, and it is the gold standard for COVID-19 diagnosis. The results for this nucleic acid test might take up to 3.5 hours, and the increased complexity of viral RNA extractions and PCR reaction setups require highly trained laboratory personnel. The COVID-19 IgM/IgG antibody rapid test is a qualitative test for COVID-19 IgM and IgG antibodies. It is less complex and can provide results in less than 15 minutes. However, it should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
For more detail on these differences, read our blog post on this topic here.
Both of our tests are FDA EUA approved and CE certified.
The test on the left of this page has only been approved for use in a CLIA high or medium complexity lab environment. This certification is not usually granted to clinics or doctor’s offices. As such, only specialized facilities with the above CLIA high or medium complexity-compliant labs would be able to perform this test.
On the other hand, the test on the right of this page has been authorized for point-of-care testing. This has been clarified by the FDA to include CLIA-waived facilities. This means that any facility with a CLIA waiver, or a clinic/doctor’s office approved to use point-of-care testing, would be allowed to administer these tests.
As per FDA’s guidance on March 16th, 2020, FDA does not intend to object to the development and distribution by commercial manufacturers or development and use by laboratories of serology tests to identify antibodies to SARS-CoV-2, where the test has been validated, notification is provided to FDA, and information along the lines of the following is included in the test reports:
- COVID-19 rapid test kits have not been reviewed by the FDA.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Following-up with a molecular diagnostic testing should be considered to rule out infection in these individuals.
- Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
No, the IgM/IgG rapid test does not require any other equipment. Aurora’s COVID-19 Antibody IgM/IgG Rapid Test Kit provides all the tools you need from sample collection to result reading.
Please refer to product manual included with the test.
Yes, each test is packed separately in the box. Each test includes: COVID-19 IgM/IgG Rapid Test cassette, disposable pipet, and sample dilution buffer. Each box contains one copy of the product manual.
The minimum order is one box for either of these COVID-19 Antibody Rapid Test. This would be for 25 tests for the non-point of care test, and 20 tests for the point of care, CLIA waived test.
The time for you to receive your order will depend on a number of factors. To receive more information on shipping times and your tracking number, please give us a call at +1-604-215-8700.
The COVID-19 Antibody Rapid Test Cassette will produce results within 10 minutes of placing the blood sample in the cassette. Results will not be valid after 15 minutes.
COVID-19 IgM/IgG Rapid Test Kits are not intended for at home use. If you feel you need to be tested, please reach out to a local clinic or physician and ask them about offering this test
The earliest time for a positive antibody test result (on the IgM line) would be about 7 days after first clinical symptoms. These first clinical symptoms usually occur about 14 days after first infection.
The COVID-19 Antibody Rapid Test can only be used as a reference, please contact your local physician or hospital.
Yes, this product is now available in Canada.
Please interpret the result of rapid antigen and antibody tests within 15-30 minutes. The result is not valid if you check again in a few hours.


