Third deadly CoV outbreak
In less than 20 years, three deadly international CoV outbreaks occurred. The first one was the Severe Acute Respiratory Syndrome (SARS) CoV-1 outbreak that started in 2003. The second one was the Middle East Respiratory Syndrome (MERS) CoV occurred in 2012 and currently, we are experiencing the SARS-CoV-2 pandemic starting from 2019.

Evidence for ion channel activity in CoV proteins and potential for modulation as a therapeutic approach
COVID-19 Drug & Vaccine Development
From early 2020, several hundred pharmaceutical and biotechnology companies, university research groups, and health organizations started developing therapeutic candidates for COVID‑19 disease. Considerable attention has been made to spike proteins on the surface of the virus. While the role of other proteins with ion channel activity is less clear. Sequencing of the virus genome revealed the presence of several envelope E proteins and putative ion channel genes. Since then numerous academic groups have begun to study and target COVID-19 viral ion channels (viroporins). Interestingly it has been proposed that Sars-Cov2 ion channels might have contributions to inflammation and severity of COVID‑19 disease. It has been also shown that the 2-E protein not only exists on the surface of the virus but also is overexpressed on the membranes of host cells and forms cation channels, which eventually lead to membrane rupture and tissue damage. In vitro and in vivo experiments revealed that some channel inhibitors have potent anti-SARS-CoV-2 activity and can protect the tissue against the 2-E-induced damage. Altogether the findings so far support that 2-E is a promising drug target against SARS-CoV-2 and forms a type of pH-sensitive cation channel that functions as ion channels in the viral membranes, as well as host cells. Aurora’s series of automated Ion Channel Readers (ICRs) obtain high throughput activity measurements for ion channels by measuring the absolute concentrations of cytosolic and extracellular ions. This technique is independent of and complementary to methods that rely on voltage manipulation and could be used for screening of compounds used as potential therapeutics.
COVID‑19 drug and vaccine development is the procedure of developing preventative therapeutic drugs and vaccines that would alleviate the severity of COVID‑19 disease.
As it is acknowledged Ion channels are one of the major drug targets that account for 13 % of FDA drugs. In the case of the Covid-19 outbreak, similar to other viral infections there are two therapeutic methods for targeting ion channels:
1) Inhibition of host ion channels with multiple ion channel modulators that have been repeatedly shown to affect virion entry and endosomal fusion.
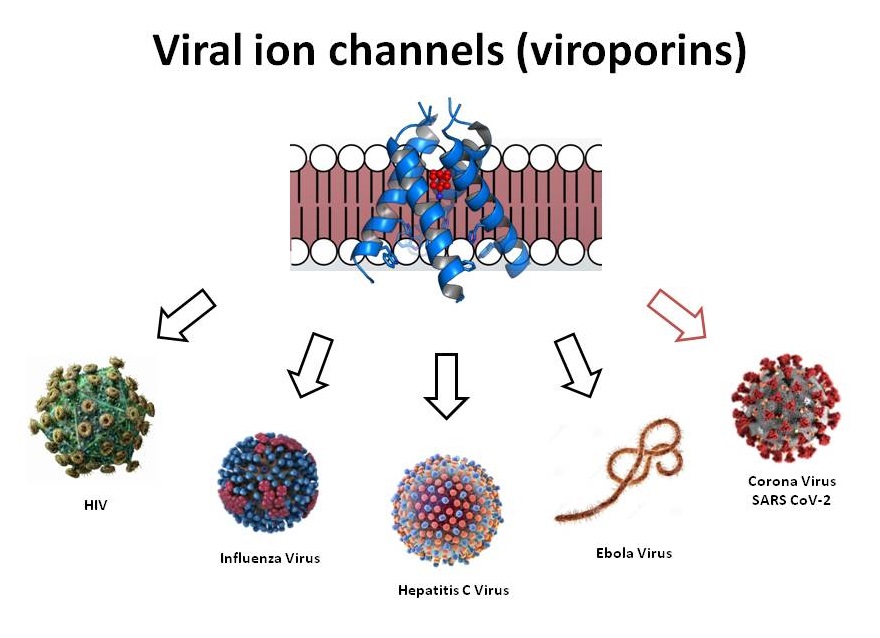
2) Identification of viral ion channels that allows screening for novel virus-specific channel modulators, given the lack of homology between the viral and human ion channels
Targeting viral Proteins with Ion Channel Activity
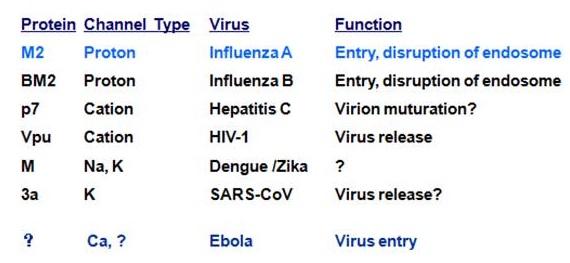
Considering the existing knowledge, as shown in the table below by Ling Chen, State Key Lab of Respiratory Disease (SKLRD), Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou Medical University, SARS CoV protein 3a (with K channel activity) was used a therapeutic target.
Potential antiviral targets
Influenza M2 was shown as a target for anti-influenza A drug discovery and M2 blockers (Amantadine & Rimantadine) were developed. Hepatitis C virus p7 Ion channel protein was also shown as a potential antiviral target. Since the initial characterization of influenza M2, viral proteins which form ion channels themselves (viroporins) or which can modulate host cell ion channel function (e.g. HIV-1 Vpu), have been reported in a variety of virus species, and repeatedly proposed as potential antiviral drug targets.
Coronaviruses spherical particles
Coronavirus with a diameter of ~125 nm is enclosed in an envelope bilayer having membrane (M), envelope (E) & spike (S) structural proteins. Membrane fusion between S protein generates the surface spikes that mediate receptor binding and the virus and the host cell. Inside the viral envelope, nucleocapsid (N) proteins stabilize the single-stranded RNA genome. E and M proteins maintain the envelope shape. Many studies have established the relevance of the E protein & protein 3a as fundamental pro-inflammatory SARS-CoV virulence factors. Additional studies have suggested that the inflammatory properties of E and 3a are related to their induction of ion conductances in membranes, i.e. that they are ion channel proteins.
Viroporins, integral viral membrane ion channel proteins
Considering the coronavirus family, ion channels mainly operate at several steps in the viral life cycle including regulation of replication compartment, viroplasm formation, virion budding, and viral infection. CoVs also code for their own ion channels. The SARS-CoV ORF3a was found to function as a potassium-specific channel promoting virus release. ORF3a & E are potential ion channels for SARS-CoV/SARS-CoV-2 as well and were proposed as candidates of drug targets. SARS-CoV E protein has a lumenal N-terminus & a cytoplasmically oriented C-terminus, a champagne flute shape structure, with the wider opening facing the cytoplasmic side. At the moment good channel inhibitors are lacking and their availability will help to reveal the specific role these channels play in the life cycle of these viruses.
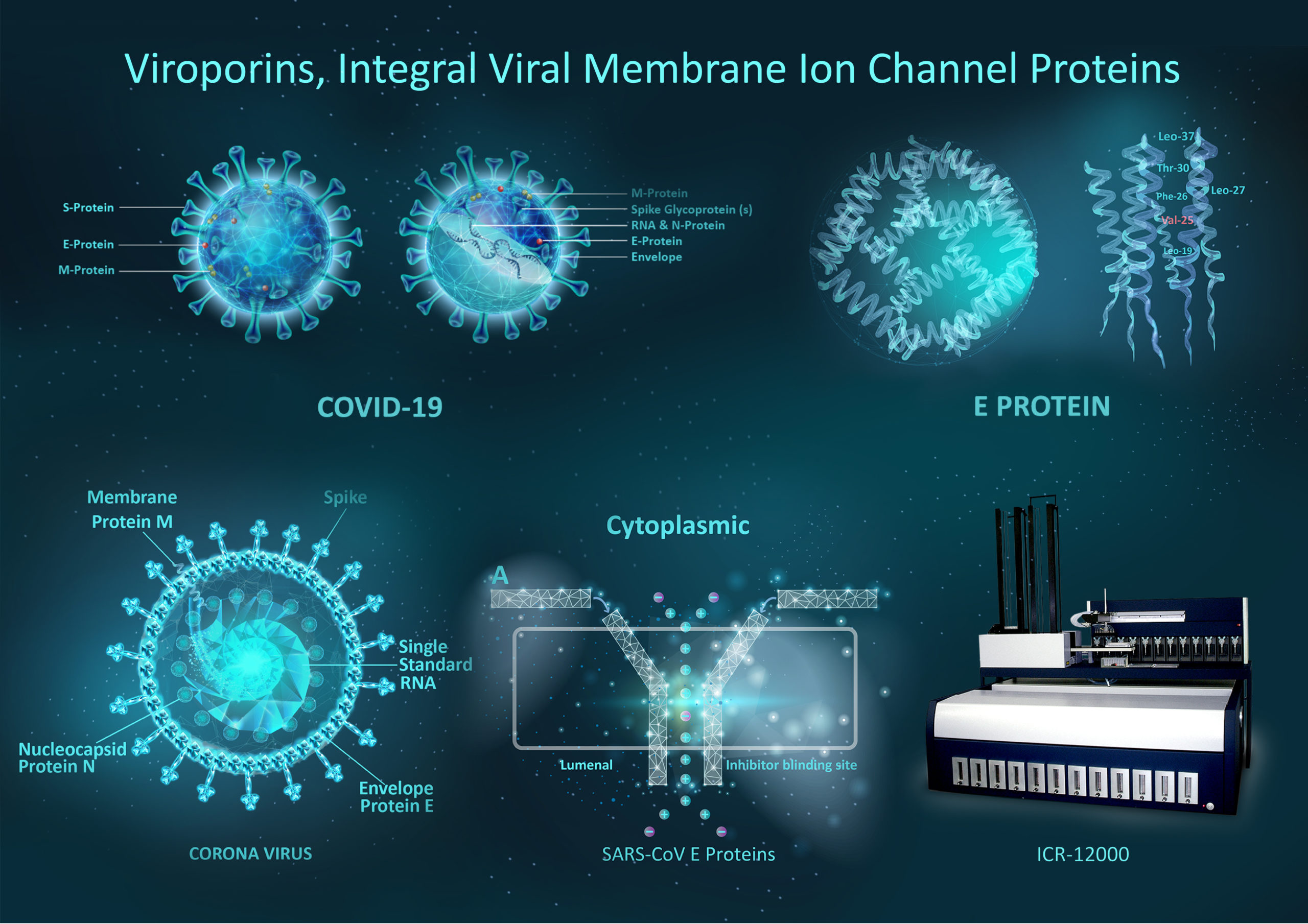
Viral ion channels forming proteins (viroporins) also identified in Severe Acute Respiratory Syndrome (SARS)-CoV-2 and exhibit low degree of homology with ion channels of prokaryotic or eukaryotic origin. Although their transmembrane (TM) regions do bear some resemblance to the corresponding regions of ion channels of higher organisms. Lack of homology between CoV viroporins and human ion channels provides the potential for selective modulation with small molecule or biologic therapies. Aurora’s Ion channel reader (ICR technology) allows for the high throughput screening of novel channel modulators.
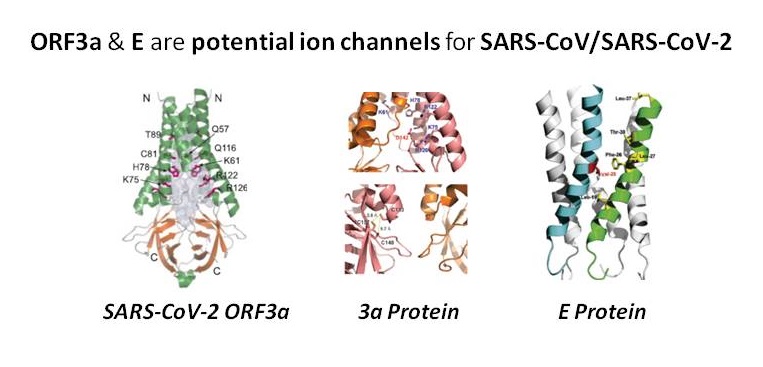
ORF3a has been proposed as candidates of drug targets but critical assessment of the current evidence for ion channel activity of SARS-CoV E protein, protein 3a, and protein 8a, all need potential studies to clarify existing uncertainties. 3a for example was first claimed to be a K+ selective channel after reconstitution into synthetic liposomes. The published data indicated that reconstituted 3a protein generates a non-selective cationic channel potentially with a large pore.
Electrophysiological characterization of E Proteins
Of all coronavirus proteins, E is the least understood. Functionally the E protein is involved in viral assembly, release, and pathogenesis. Small size and overall hydrophobicity prompted suggestions that coronavirus E proteins might be viroporins. Synthetic SARS-CoV E, or E proteins from mouse hepatitis virus (MHV)/infectious bronchitis virus (IBV) was reconstituted into artificial bilayers. Reports demonstrated that these proteins cause fluctuating currents and indistinct gating events. Also, different apparent ion permeability series as shown below were observed:
a-CoV HCoV-229 (K+ > Na+ > Cl−)
MHV (b-CoV) and IBV (g-CoV) (both Na+ > K+ > Cl−)
Attenuated viruses lacking the E protein have even been suggested to serve as vaccine candidates. E proteins from several coronaviruses including SARS-CoV-1, MERS coronavirus, human coronavirus, mouse hepatitis virus, and infectious bronchitis virus were shown to possess cation-selective channel activity that may be blocked by compounds like Hexamethylene amiloride.
Aurora’s Ion Channel Readers and HTS Assays:
Aurora Biomed’s automated ICRs (Ion Channel Readers) combine the precision and sensitivity of atomic absorption spectroscopy (AAS) with patented microsampling processes and liquid handling technologies to create a high-throughput screening solution for ion channel researchers that fills gaps which automated patch-clamp cannot. By detecting ion channel activity using atomic absorption spectroscopy and flux assays, the ICR series of automated Ion Channel Readers can analyze ion channels regardless of electrogenicity. ICR technology can be used with both voltage-gated (e.g., hERG, Kv1.1, Nav1.5) and ligand-gated (e.g., KATP, nAChR) channels, as well as with ion pumps and transporters (e.g., Na+/K+-ATPase), allowing researchers to accelerate drug development for the treatment and prevention of diseases associated with these key membrane transport proteins. Our ICR series of automated Ion Channel Readers obtain high throughput activity measurements for these and other channels and transporters by measuring the absolute concentrations of cytosolic and extracellular ions. This technique is independent of and complementary to methods that rely on voltage manipulation.
Aurora’s non-radioactive flux assays eliminate the need for radioisotopes, making your ion channel screens safer and more environmentally friendly while negating any work restrictions posed by Rb’s short half-life. The AAS-based screening technology means that there is no need to worry about quenching effects associated with fluorescence.


