
Used to purify samples and isolate elements, various methods of extraction are extremely important in the chemical analysis process. These processes can be quite complex, but it is important to understand these methods and the role they play in many industries. Below is a breakdown of the processes used in liquid-liquid extraction and solid-phase extraction as well as their applications across several industries.
Liquid-Liquid Extraction
liquid-liquid extraction, also known as solvent extraction or partitioning, is a process used across many industries. This process uses two immiscible liquids, typically one aqueous and one organic, in order to separate compounds. Lab techs will transfer a solute from one solvent to another. To complete this transfer, the two liquids must make contact and mix. Then comes phase separation, in which the two immiscible liquids become separate once again.
The process begins with a solvent and a feed solution which contains the solute for extraction. When choosing a solvent, there are a few factors to consider in order to facilitate the most valuable results. First, one must consider the ease with which the solvent can extract the solute from the feed solution. The chosen solvent should also be noncorrosive and possess good recoverability. Additionally, the interfacial tension between the phases should be precise; if the tension is too low, disengagement becomes increasingly difficult.
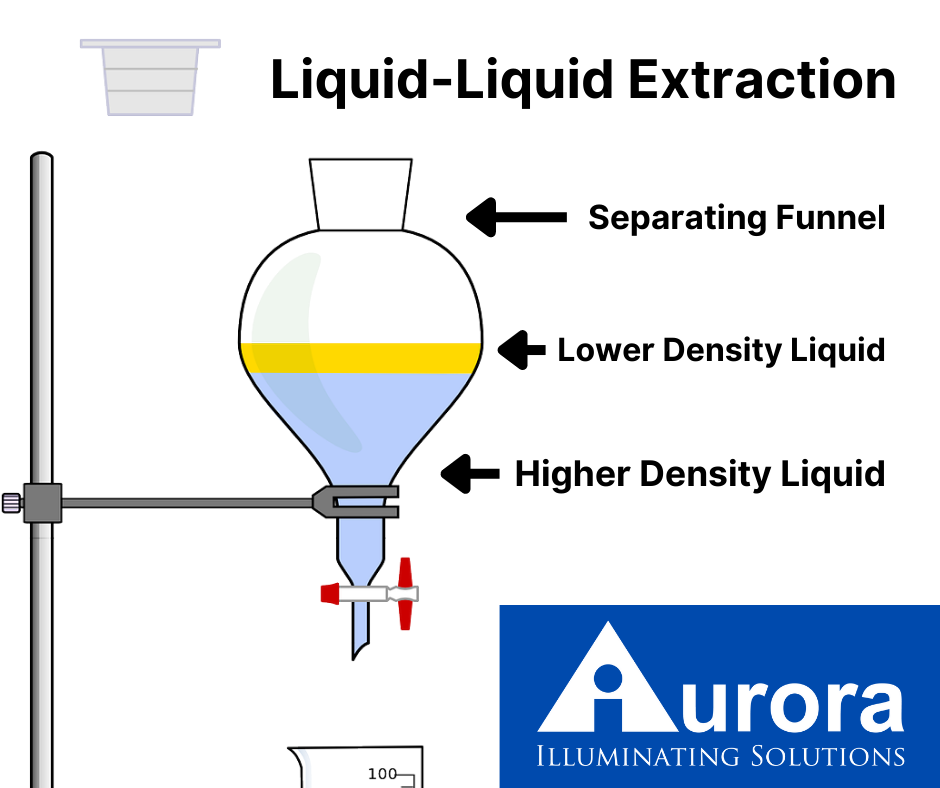
A lab tech will then place the two liquids in a container and shake or stir them together. This process allows the molecules to dissolve into the chosen solvent. Many professionals refer to this as partitioning. The phases can then settle, with the solute now residing in the aqueous solvent, now called the extract. The organic solvent, now void of the solute, is a raffinate.
As we’ve stated, there are many different applications for liquid-liquid extraction, leading to several different techniques for the process. In smaller-scale chemical labs, it is a common procedure to use a separatory funnel to produce batch single-stage extractions. This process typically includes dispersive liquid-liquid microextraction and direct organic extraction, processes that are particularly useful in the extraction of proteins and identification of pesticides.
Larger industries, however, typically use multistage countercurrent continuous processes, such as the extraction of lanthanides from metals. As this process requires many small extraction stages, it can be quite time-consuming and demands the utmost precision and accuracy. Automated liquid-liquid extraction workstations expedite the process, reducing the risk of human error and allowing lab technicians to focus their attentions elsewhere. Professionals can equip these workstations with additional attachments, such as a centrifuge used to mix and separate the liquids, to create a more efficient and precise process.
Solid Phase Extraction
Solid-phase extraction differs from liquid-liquid extraction in the fact that the separation of an analyte is achieved through interaction with a solid stationary phase. It offers a range of benefits over liquid-liquid extraction such as the removal of possible emulsion formation and the ability for quantitative recovery. It is available in three main types: normal phase, reversed phase, and ion exchange – that are typically useful for polar, non-polar, and charged compounds, respectively. Solid-phase extraction typically occurs in the following steps.
First, it must determine which type of stationary phase is suitable for the isolation of the desired analyte. A lab tech will then condition and equilibrated the corresponding cartridge with the appropriate mobile phase. The sample is then dissolved in solvent and loaded onto the stationary phase. Either positive or negative pressure is applied to the cartridge in order to push/pull the sample through the stationary phase. As the sample passes through the stationary phase, different compounds will elute at different rates due to their affinity for the stationary phase. Finally, the analyte is isolated by removal of the eluent, leaving the desired final product.
In some instances, one may have to carry out this process several times in order to completely extract the analytes from the sample. This can be an incredibly time-consuming process and can become a major pain point for laboratory technicians. However, automated workstations, such as those in our VERSA series, completely automate this intensive process. Utilizing high-end robotic technology, these workstations streamline the process by reducing the risk of human error and providing extremely consistent, accurate, and precise results.
To learn more about how automated liquid handling equipment can make the extraction process more efficient and accurate, check out our beginner’s guide to automated liquid handling.
Applications
Automated liquid handling has streamlined the process of solid-phase and liquid-liquid extractions and has many applications across various industries. In fact, according to a 2018 study “The Global Automated Liquid Handling Market size is expected to reach $1.05 billion by 2024, rising at a market growth of 7.8% CAGR during the forecast period.” Below are just a few of the industries that use automated liquid handling equipment in their solid-phase and liquid-liquid extractions to increase efficiency, precision, and accuracy.
Agriculture
Farming and agriculture have been important industries in the United States for many years, with their success impacting people all around the world. Recently, many farmers have turned to liquid-liquid extractions, in order to better keep up with new regulations and the ever-increasing demands of business and innovation. In this industry, professionals use liquid-liquid extractions to identify disease-resistant genes in crops, improving crop yield and reducing the risk of crop loss. Additionally, agriculturists can use this technology to predict offspring, allowing farmers to optimize future livestock and crop yields.
Food Safety
Similar to its use in the agriculture industry, the food safety industry uses solid-phase extractions and liquid-liquid extractions to identify herbicides pesticides, food toxins, and other chemicals that may be harmful when ingested. These processes can also detect allergens and pathogens, ensuring that the food we eat is both healthy and safe. Professionals will also use solid-phase extraction to extract caffeine from coffee beans or to extract oil from oilseeds and sugar from sugar cane.
Pharmaceutical
The pharmaceutical industry is constantly changing, with scientists discovering new medications every day. Automated liquid handling equipment allows pharmacists to stay abreast of the latest innovations in the pharmaceutical industry. When used in the pharmaceutical industry, liquid-liquid extraction is able to isolate and identify proteins, antibiotics, amino acids, and enzymes. Additionally, professionals can use liquid-liquid extraction to extract essential oils, which manufacturers can use in fragrances, flavoring, and pharmaceuticals. Secondary metabolites, compounds can be extracted from plants to study their mode of action.





